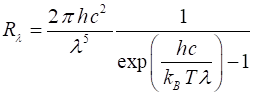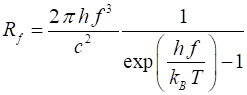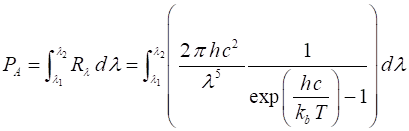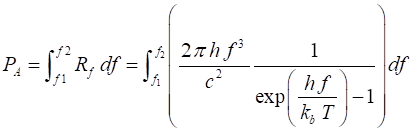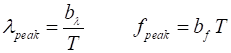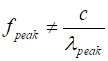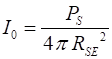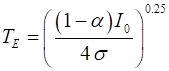|
SOLAR
RADIATION MODELLING BLACKBODY
RADIATION Ian Cooper |
|
DOWNLOAD
DIRECTORIES FOR MATLAB SCRIPTS tpSun.m Simulation
of the electromagnetic radiation emitted from the Sun. The Script can be used
to create colour spectrums of the radiation emitted
from the Sun by calling the Script Colorcode.m. solarData.mat Solar spectrum data for the effects of the atmosphere of the radiation
reaching the ground: AM0 and AM1.5. simpson1d.m Function
to evaluate the area under a curve using Simpson’s 1/3 rule. ColorCode.m Function
to return the appropriate colour for a wavelength
in the visible range from 380 nm to 780 nm. |
|
BLACKBODY
RADIATION PARTICLE NATURE OF
ELECTROMAGNETIC RADIATION The
goal of this article to investigate the solar
spectrum: the Sun as a blackbody and the calculation of the
intensity of solar irradiance at the Earth's surface. The wave nature of
electromagnetic radiation is demonstrated by interference phenomena. However,
electromagnetic radiation also has a particle nature. For example, to account
for the observations of the radiation emitted from hot objects, it is
necessary to use a particle model, where the radiation is considered to be a
stream of particles called photons.
The energy of a photon, E is (1) The
electromagnetic energy emitted from an object’s surface is called thermal radiation and is due to a decrease
in the internal energy of the object. This radiation consists of a continuous
spectrum of frequencies extending over a wide range. Objects at room
temperature emit mainly infrared and it is not until the temperature reaches
about 800 K and above those objects glows visibly. A blackbody is an object that
completely absorbs all electromagnetic radiation falling on its surface at
any temperature. It can be thought of as a perfect absorber and emitter of
radiation. The power emitted from a blackbody, P is given by
the Stefan-Boltzmann
law and it depends only on the surface area of the emitter, A and its surface temperature, T (2) A more
general form of equation (2) is (2) where e is the emissivity of the object. For a blackbody, e = 1. When e < 1 the object is called a graybody and the object is not a perfect emitter and
absorber. The amount
of radiation emitted by a blackbody is given by Planck’s radiation law and is
expressed in terms of the spectral exitance for wavelength or frequency
Rl or Rf respectively (4) or (5) The spectral exitance
is also called the spectral irradiance [W.m-2.m-1]. In the
literature, many different terms and symbols are used for the spectral
exitance. Sometimes the terms and the units given are wrong or
misleading. Note units for
equation 1: The units in the denominator
should be thought of in terms of m2.m where the
first factor m2 corresponds to the surface area of the
blackbody and the second unit of length m corresponds to the
wavelength of the emitted light. The power radiated per unit surface of a blackbody, PA within
a wavelength interval or bandwidth, (l1, l2) or frequency interval or
bandwidth (f1, f2) are given by equations
6 and 7 (6) and (7) The equations
6 and 7 give the Stefan-Boltzmann law (equation 2) when the bandwidths extend
from 0 to ¥. Wien’s Displacement law states
that the wavelength lpeak corresponding to the peak of
the spectral exitance given by equation 4 is inversely proportional to the
temperature of the blackbody and the frequency fpeak for the
spectral exitance peak frequency given by equation 5 is proportional to the
temperature (8)
The peaks
in equations 4 and 5 occur in different parts of the electromagnetic spectrum
and so (9) The
Wien’s Displacement law explains why long wave radiation dominates more
and more in the spectrum of the radiation emitted by an object as its
temperature is lowered. When
classical theories were used to derive an expression for the spectral
exitances Rl and Rf, the power
emitted by a blackbody diverged to infinity as the wavelength became shorter
and shorter. This is known as the ultraviolet
catastrophe. In 1901 Max Planck proposed a new radical idea that
was completely alien to classical notions, electromagnetic energy is quantized. Planck was able to derive the equations 4
and 5 for blackbody emission and these equations are in complete agreement
with experimental measurements. The assumption that the energy of a system varies
in a continuous manner, i.e., (take any arbitrary close consecutive values
fails. Energy can only exist in integer multiples of the lowest amount or
quantum, h f. This step marked the very beginning of modern quantum theory. A summary of the physical quantities, units and values of constants used in the description of the radiation from a hot object. |
Variable |
Interpretation |
Value |
Unit |
|
E |
energy of photon |
|
J |
|
h |
Planck’s constant |
6.62608´10-34 |
J.s |
|
c |
speed of electromagnetic radiation |
3.00x108 |
m.s-1 |
|
f |
frequency of electromagnetic
radiation |
|
Hz |
|
l |
wavelength of electromagnetic
radiation |
|
|
|
T |
surface temperature of object |
|
K |
|
A |
surface area of object |
|
m2 |
|
s |
Stefan-Boltzmann constant |
5.6696´10-8 |
W.m-2.K-4 |
|
P |
power emitted from hot object |
|
W |
|
e |
emissivity of object’s
surface |
|
|
|
Rl |
spectral exitance: power radiated
per unit area per unit wavelength interval |
|
(W.m-2).m-1 |
|
Rf |
spectral exitance: power radiated
per unit area per unit frequency interval |
|
(W.m-2).s-1 |
|
kB |
Boltzmann constant |
1.38066´10-23 |
J.K-1 |
|
bl |
Wien constant: wavelength |
2.898´10-3 |
m.K |
|
bf |
Wien constant: frequency |
2.83
kB T / h |
K-1.s-1 |
|
lpeak |
wavelength of peak in solar
spectrum |
5.0225´10-7 |
m |
|
RS |
radius of the Sun |
6.96´108 |
m |
|
RE |
radius of the Earth |
6.96´106 |
m |
|
RSE |
Sun-Earth radius |
6.96´1011 |
m |
|
I0 |
Solar constant |
1.36´103 |
W.m-2 |
|
a |
Albedo of Earth’s surface |
0.30 |
|
|
THE SUN AS A BLACKBODY
The Sun can
be considered as a blackbody, and the total power output of the Sun PS can be estimated by using the Sefan-Boltzmann law, equation 2, and by finding the area
under the curves for Rl and Rf using equations 6 and 7. From observations on the Sun, the peak
in the electromagnetic radiation emitted has a wavelength, lpeak = 502.25 nm (green). The temperature of the Sun’s surface
(photosphere) can be estimated from the Wien displacement law, equation 8. The distance
from the Sun to the Earth, RSE can be used to estimate of the
surface temperature of the Earth TE if there
was no atmosphere. The intensity of the Sun’s radiation reaching the
top of the atmosphere, I0 is known as
the solar constant (10) The power
absorbed by the Earth, PEabs is (11) where a is the
albedo (the reflectivity of the Earth’s surface). Assuming the Earth
behaves as a blackbody then the power of the radiation emitted from the Earth,
PErad is (12) It is known
that the Earth’s surface temperature has remained relatively constant
over many centuries, so that the power absorbed and the power emitted are
equal, so the Earth’s equilibrium temperature TE is (13) The Script tpSun.m can be used
to calculate and plot details of the Sun and Earth as blackbodies. The
results of the computation are displayed in the Command Window and Figure
Windows. Plots of the spectral
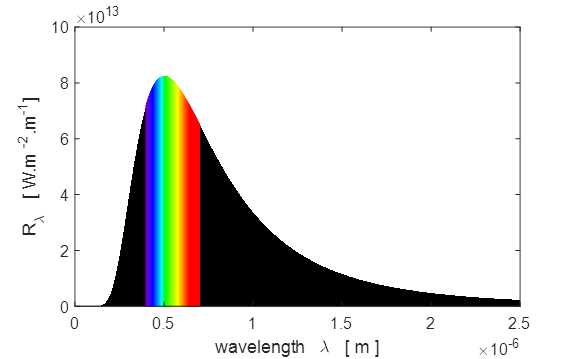
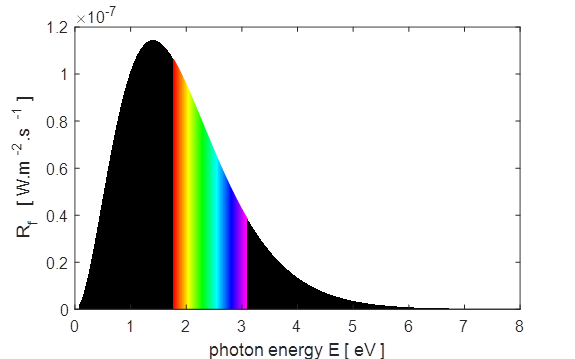
exitance curves for the Sun as shown in figure 1.
Fig.
1. Plots of the spectral
exitance curves as a function of wavelength and photon energy Matlab screen
output for tpSun.m Sun:
temperature of photosphere, T_S = 5770 K Peak
in Solar Spectrum Theory: Wavelength at peak in
spectral exitance, wL = 5.02e-07 m Graph: Wavelength at peak in spectral
exitance, wL = 5.04e-07 m Corresponding frequency, f =
5.95e+14 Hz Theory: Frequency at peak in
spectral exitance, f = 3.39e+14 Hz Graph: Frequency at peak in spectral
exitance, f = 3.40e+14
Hz Corresponding wavelength, wL = 8.82e-07 m Total
Solar Power Output P_Stefan_Boltzmann
= 3.79e+26 W
P(wL)_total =
3.77e+26 W
P(f)_total
= 3.79e+26
W IR
visible UV P_IR = 1.92e+26 W Percentage IR radiation = 51.0 P_visible
= 1.39e+26 W
Percentage visible radiation =
36.8 P_UV = 4.61e+25 W Percentage UV radiation = 12.2 Sun
- Earth Theory: Solar constant I_O =
1.360e+03 W/m^2 Computed: Solar constant I_E = 1.342e+03 W/m^2 Surface temperature of the
Earth, T_E = 254 K
Surface temperature of the
Earth, T_E = -19 deg C M-script
highlights 1 Suitable values for the wavelength and frequency integration limits
for equations (6) and (7) are determined so that the spectral exitances at
the limits are small compared to the peak values. 2 The Matlab
function area is
used to plot the spectral exitance curves, for example, in plotting the Rl curve: h_area1
= area(wL,R_wL); set(h_area1,'FaceColor',[0
0 0]); set(h_area1,'EdgeColor','none'); 3 The color for the
shading of the curve matches that of the wavelength in the visible part of
the spectrum. A call is made to the function ColorCode.m to assign a color for a given
wavelength band. For the shading of the Rl curve: thisColorMap = hsv(128); for
cn = 1 : num_wL-1 thisColor = ColorCode(wL_vis(cn)); h_area = area(wL_vis(cn:cn+1),R_wL_vis(cn:cn+1)); set(h_area,'FaceColor',thisColor); set(h_area,'EdgeColor',thisColor); 4 Simpson’s
1/3 rule is used for the numerical integration (simpson1d.m) to find the area under the spectral
intensity curves. For the Rl curve, the total power radiated by the Sun: P_total = A_sun * simpson1d(R_wL,wL1,wL2); 5 The
peaks in spectral intensities are calculated using Matlab logical functions: wL_peak_graph
= wL(R_wL == max(R_wL)); f_peak_graph
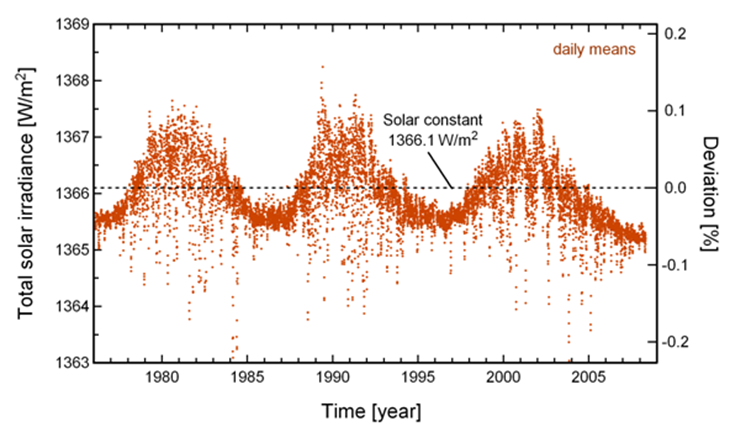
= f(R_f == max(R_f)); SOLAR CONSTANT The flux of
solar radiation energy that arrives at the outermost layers of the atmosphere
is called the total solar irradiance.
It varies slightly in an annual cycle because the Earth revolves around the
Sun on an elliptic orbit (figure 2). The
data show very slight variations (by about 0.1%) due to the 11-year solar
cycle. The long-term average of the total solar irradiance is called the solar constant
Fig.
2. Each red dot plots the
daily mean of the total solar irradiance measured at vertical incidence above the
Earth’s atmosphere by satellites. The dashed line plots the long-term
average of the solar constant The value
of the solar constant Theory: Solar constant I_O =
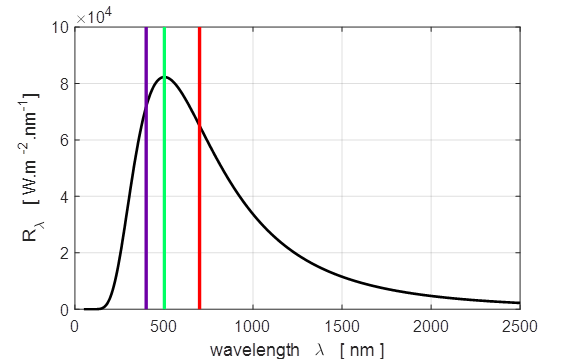
1.360e+03 W.m-2 Computed: Solar constant I_E = 1.342e+03 W.m-2 The extraterrestrial (AM0) solar spectrum The energy of sunlight is called intensity
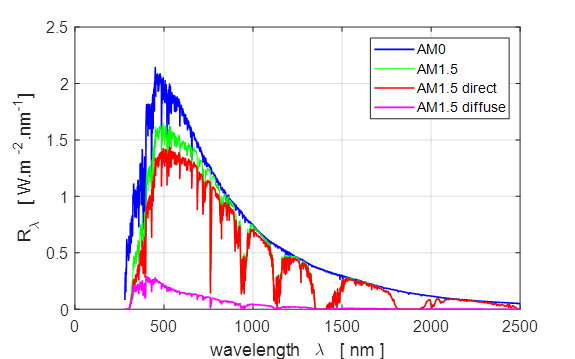
or irradiance. Figure 3
shows the blackbody curve for the Sun at a temperature of 5770 K, and the
spectral exitance curves measured above the top regions of the Earth’s
atmosphere (AMO blue line), and the curves
for AM1.5 (AM1.5 green line, direct radiation red line, diffuse
radiation magenta line). The acronym AM0 stands
for the spectrum for air mass zero, meaning that the spectrum was measured
with no air between the Sun and the detector. AM
Fig, 3. Blackbody curve for the Sun at a
temperature of 5770 K (top plot). The spectral irradiance curves AM0 and
AM1.5 (bottom plot). The data for
the curves in the bottom plot was downloaded as an Excel file by searching solar
spectral irradiance data ASTM G-173. tpSun.m Data file SolarData.mat
Note sure why the vertical
scale of the top and bottom plots are so different
in magnitude? The Earth's atmosphere has a large impact upon the solar
radiation reaching the surface of the Earth. The AM0 spectrum applies only to
the top of the atmosphere. At the surface of the Earth, the solar spectrum is
very different and cannot be approximated by the AM0 spectrum or the black
body spectrum. The terrestrial solar spectrum is variable, as we know from
daily life: the Sun changes color and intensity all the time.
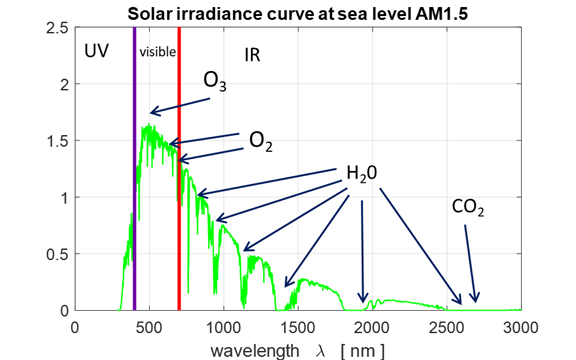
The following are the main mechanism for this spectral change: · Absorption of radiation due to molecules and particles in the
Earth atmosphere: water vapor, carbon dioxide, methane and other greenhouse gases
mostly in the infrared region and by ozone in the visible and ultraviolet
portion of the spectrum. · Absorption and scattering by clouds. · Scattering of radiation by molecules and particles in the
atmosphere. Scattering of mainly blue light by dust and ice particles in the
atmosphere, giving rise to direct and diffuse components. · Reflection of radiation from the Earth surface. ·
The mass of
air the light must travel through which depends on the elevation of the Sun
over the horizon. The
Fig. 4. Simply
diagram of the absorption of solar radiation by greenhouse gases. |